Abstract
Introduction: Global application of bone marrow transplant (BMT) for sickle cell disease (SCD) requires a protocol that addresses the core limitations of this approach - namely donor availability, graft-versus-host disease (GVHD) and engraftment. To meet these demands, we developed the Vanderbilt Haploidentical Learning Collaborative across 10 countries and 22 sites with a common related HLA-haploidentical (haploBMT) reduced intensity platform. Our phase II trial (ClinicalTrials.gov identifier NCT01850108) was successfully employed with a probability of overall survival of 96.7% (95% CI 87.1- 99.2%) at one year, but a high rate of graft failure (GF) in pediatric patients (<18 years), prompting us to perform an in-depth analysis of engraftment kinetics.
Methods: Seven Institutional Review Board approved sites enrolled patients undergoing a haploBMT for SCD from 8/14/2014-2/28/2022. Common conditioning included: thymoglobulin 4.5 mg/kg, thiotepa 10 mg/kg, fludarabine 150 mg/m2, cyclophosphamide 29 mg/kg and TBI 2Gy. GVHD prophylaxis included post-transplant cyclophosphamide 100 mg/kg, mycophenolate mofetil, and sirolimus (de la Fuente et al. BBMT 2019). Hydroxyurea and hypertransfusion to lower the percentage hemoglobin S <30% for at least 60 days pre-conditioning was variably employed. Primary GF was defined as <5% donor myeloid/lymphoid or whole blood (WB) chimerism at one-month (M1) post-haploBMT. Secondary GF was defined <5% donor myeloid/lymphoid or WB chimerism with prior documentation of >5% donor cells at M1. Categorical variables were compared using Fisher's exact test. Continuous variables were compared using Wilcoxon rank sum test.
Results: A total of 41 pediatric and 39 adult subjects were enrolled for data analysis. GF was only noted in the pediatric cohort with 4 (10%) and 6 (15%) developing primary and secondary GF, respectively. Primary GF was associated with lack of a hypertransfusion pre-conditioning (0% vs 54%) and CMV reactivation (75% vs 21%) compared to engrafted patients.
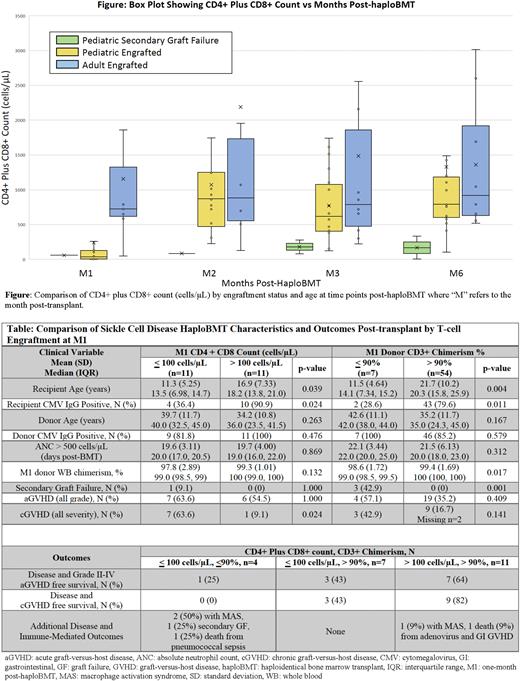
For secondary GF, we evaluated the mechanisms of prolonged tolerance including early donor T-cell engraftment. Although our data was limited by only 28% (n=22) having lymphocyte subsets at M1, pediatric patients had lower T-cell counts (median 45.0 cells/µL, IQR: 6.50-118) compared to adults (median 723 cells/µL, IQR 618-1325) (Figure). Older recipient age and positive recipient CMV status were the only variables significantly associated with both CD4+ plus CD8+ count >100 cells/µL and donor CD3+ chimerism >90% (Table). Pediatric T-cell engraftment matched adult values at later time points except in patients with secondary GF and improvement in T-cell counts above 500 cells/µL at M2 was associated with a higher TNC dose (median 4.57x108 cells/µL, IQR 2.86-5.75 vs 1.74x108 cells/µL, IQR 1.55-2.24).
We next evaluated the impact of low donor T-cell engraftment at M1. Patients with low donor T-cell engraftment had significantly higher rates of immune-mediated complications including secondary GF (CD3+ chimerism <90%: 42.9% vs 0%, p=0.001) and chronic GVHD (CD4+ plus CD8+ <100 cells/µL: 63.6% vs 9.1%, p=0.024). Incidence of viral reactivation was not different between cohorts. Disease and GVHD-free survival positively trended with donor T-cell engraftment (Table), and patients with both CD4+ plus CD8+ count <100 cells/µL and donor CD3+ chimerism <90% at M1 had severe immune-mediated complications, implicating an association of inadequate immune control with poor early donor T-cell engraftment.
Conclusions: This analysis illustrates the importance of early T-cell engraftment in developing bi-directional immune tolerance following haploBMT. Although our study identified recipient age and CMV status as important factors in T-cell engraftment, the mechanism behind these factors, such as age-associated differences in drug metabolism and the role of CMV in promoting donor T-cell expansion, and how donor T cells promote tolerance require additional investigation. Our study demonstrates the importance of monitoring T-cell counts and sorted chimerism early post haploBMT and raises the question of how best to intervene in patients with low donor T-cell engraftment at M1. Our consortium plans to prospectively evaluate immune tolerance, including regulatory T-cell dynamics and impact of immune interventions at M1, to answer these important questions.
Disclosures
Connelly:X4: Consultancy, Current Employment; Horizon: Consultancy, Ended employment in the past 24 months; Sobi: Consultancy, Ended employment in the past 24 months. Nur:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Gatwood:Jazz Pharmaceuticals: Speakers Bureau; Kite Pharma: Speakers Bureau; sanofi: Speakers Bureau; AstraZeneca: Research Funding. Kitko:Horizon Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal